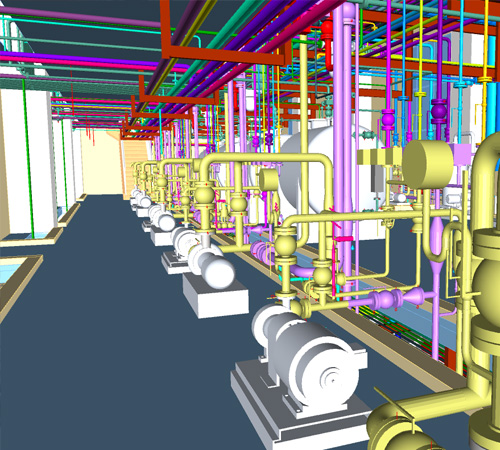
Aevitas has conceptualised and engineered facilities in API, HPAI, Peptides and Sterile API for various pharmaceutical companies. The plant designs meet NBC, Factory Acts, ISPE Baseline, ICH Q7 & ICH Q 9, FDA and WHO guidelines and safety guidelines of OSHA, NFPA etc.
Aevitas’s engineers are well versed with the containment and closed transfer needs as per the potency level of the product handled.
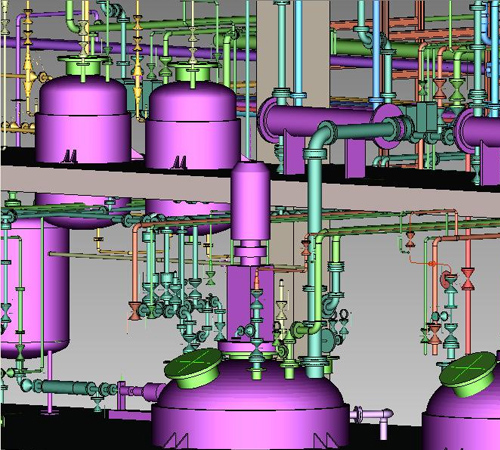
Our experience includes special reactions like hydrogenation, cryogenic reaction, sodium metal reaction, nitration, bromination and many more.
We have engineered equipment like reactors, distillation columns, short path distillation units, continuous & batch centrifuges, rotary pressure filter, spherical dryer, fluidized bed dryer, agitated thin film dryers, agitated Nutsche filter dryer, spray dryer, scrubber, jet mill, powder processing & transfer systems, conventional utilities, single fluid systems and purified water systems.
Our team of engineers have been involved in the engineering, construction and commissioning of API manufacturing plants as well as solvent recovery systems.